INTRODUCTION
Primary care physicians (PCPs) are responsible for the management of more neurological visits than neurologists.1 Recognition of neurological symptoms in the primary care setting is of the upmost importance in terms of patient management.2 In many states, chiropractors are considered PCPs, with some states requiring them to undergo additional training in minor surgery and obstetrics before obtaining a license. As PCPs, certain expectations exist when formulating an accurate and timely differential diagnosis. This case report discusses a case of delayed recognition of neurological symptoms in a patient with weakness and numbness symptoms in the left side of the face, left upper extremity and left lower extremity, following an incident of increased hemodynamic stress during a bench press maximum repetition load.
The initial differential diagnosis made by the patient’s healthcare team included radiculopathy attributed to cervical and lumbar disc herniation. The original diagnosis does not address the chief complaint of left face numbness and weakness but could explain the upper and lower extremity symptomatology. The patient sought out a second opinion due to a lack of symptom improvement 2 months after onset of symptoms. After a thorough examination and prompt referral, a differential diagnosis of hemorrhaged cerebral cavernous malformation (CCM) versus posterior cerebral artery aneurysm (PCAA) was given.
Both CCMs and PCAAs are conditions that can go undiagnosed,3 both of which can be asymptomatic in presentation or cause aggravating neurological symptoms including death when hemorrhaged.4–6 Unruptured cerebral aneurysms are present among 2-6% of the population,7 while asymptomatic CCMs are present in about 0.16% of the population.8
The objective of this case report is to showcase a rare and difficult case with different working diagnoses, to bring awareness of the importance of early neurological symptom recognition, and to hopefully by reviewing the characteristics of both conditions, help determine a likely diagnosis.
Case REPORT
A 24-year-old male sought care for weakness and numbness on the left side of his face, left upper and left lower extremities. During the history, he related that the symptoms started 2 months earlier while performing a personal record bench press repetition of 250 lbs, when he felt sudden dizziness and numbness throughout his body. The next day after the mechanism of injury he reported left-sided numbness to the whole face, left upper and left lower extremities, inability to walk due to left leg weakness and numbness and inability to grasp a cup of water with his left hand due to weakness and numbness of his left upper extremity. The patient sought care from his primary care physician and with an orthopedic surgeon, who ordered a Cervical spine (C-spine) MRI and a Lumbar spine (L-spine) MRI without contrast.
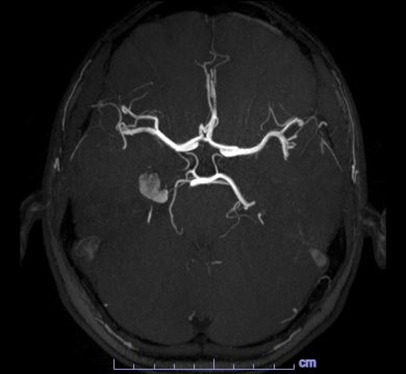
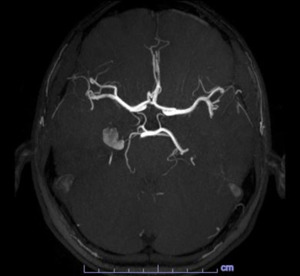
The C-spine (Fig.1) and L-spine (Fig.2) MRI results unfortunately did not explain the patient’s symptomatology.
After 2 months he felt he was not improving and sought care at the chiropractic clinic. At that time, he complained of constant numbness in his left cheek, left biceps, left wrist, left 1st and 5th metacarpals, 1st metatarsal, and the left ball of the foot. There was constant burning and tingling in the left shoulder, in the left mid-thigh, left ankle, and the 2nd and 5th metatarsals. Symptoms had improved in the last 2 months except for the ones mentioned above. After thorough evaluation of the his history, a detailed examination on the initial visit included neurological testing, range of motion (ROM), orthopedic tests, and cranial nerve examination.
The positive neurological findings included a myotome evaluation, on which T1 on the left was graded 4/5, L4-L5 on the left was graded 4/5. A dermatomal sensory evaluation on which the patient reported hypoesthesia on the left C7-T1 and left L5 dermatome. Deep tendon reflex evaluation revealed a C6 reflex graded 3+ on the left, left Achilles reflex was graded 3+. The Babinski reflex was negative, but the patient could not feel any sensation at the ball of the foot and big toe. Upon performing a cranial nerve examination, he reversed the sharp and dull sensation on the left side of his face for the Trigeminal cranial nerve 5 and he noted that there were areas of numbness in his left cheek.
Orthopedic testing revealed a positive Schepelmann’s Sign on the right for contralateral pain, which could be indicative of pleurisy or muscle sprain/strain. Wright’s test was positive on the left which was indicative of decreased radial pulse positive for thoracic outlet syndrome (TOS). There were no positive orthopedic exams for the cervical or lumbar region.
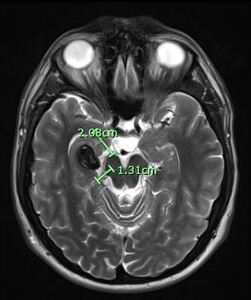
After reviewing the patient’s subjective and objective data and considering his current presentation, with upper and lower limb neurological changes, a working diagnosis was developed where a brain lesion was suspected. The patient was promptly referred to a consult with a neurologist and for an MRI with contrast of the brain. The result of the initial MRI revealed a 2.1 cm lesion within the medial aspect of the right temporal lobe (fig. 3).
Differential considerations following the initial brain MRI include a CCM with an adjacent venous anomaly or an AVM with prominent aneurysm. This MRI also revealed small T2 hyperintense lesions within the right thalamus which may represent dilated perivascular spaces or remote lacunar infarcts (fig. 4).
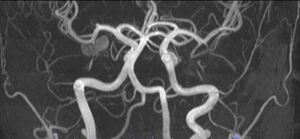
With the diagnosis not clear, an MRA with contrast was obtained the next day which revealed a 1.2 cm lesion involving the medial right temporal lobe, the radiologist favored a cavernous hemangioma but could not completely rule out an aneurysm as the lesion is very hyperintense on T1-weighted images (figs.5 and 6).
With these results he was referred for a neurosurgical consult. The neurosurgeon ordered a cerebral arteriogram and a CTA of the brain. The neuroradiologist reviewed the CTA and determined that the study demonstrated hyperdensity and calcification but that no discrete aneurysm could be identified. After reviewing the results of these studies, the neurosurgeon and neurologist concluded that the patient had a thrombosed PCA aneurysm, as the lesion is adjacent to the P2 segment of the right posterior cerebral artery at roughly the junction of the A and B portions. However, the neuroradiologist maintained his position that this lesion is more likely to be a cavernous angioma. The patient was instructed by the specialists to return to normal activity as enough time had passed without any detrimental changes and without any new symptomatology. In his last visit to the neurologist, he was prescribed Levetiracetam 500 mg due to his higher risk of seizures. The patient was than able to resume all activities, including weightlifting. hiss patchy paresthesia is still present in his left cheek, hand, and foot. All other symptoms have slowly resolved. The total time elapsed from onset to last neurological consult was 4 months.
Discussion
PCP’s have the responsibility to make the first differential diagnosis list through adequate history intake interpretation and in this case neurological exam findings.2 This case presents a challenging differential diagnosis due to the difficulty in distinguishing between a hemorrhaged cerebral cavernous malformation (CCM), an arteriovenous malformation (AVM), or a thrombosed aneurysm based on both MRI and MRA results. The neurosurgeon and the neuroradiologist involved had different opinions on the final diagnosis, illustrating the complexity of the case.
Cerebral aneurysms typically involve the weakening of the arterial wall, particularly in areas like the internal elastic lamina and the media layer, leading to the formation of aneurysms or arterial pouches.9 There are different types of cerebral aneurysms. Dissecting aneurysms typically involve the extracranial arteries while fusiform aneurysms usually involve the internal carotids and the vertebrobasilar arteries and are mostly due to atherosclerosis. Furthermore, mycotic aneurysms are due to bacterial, fungal, or viral infections. Saccular aneurysms, also known as berry aneurysms, appear at the bifurcation sites of the internal carotid, the posterior communicating artery, and basilar artery. In addition, saccular aneurysms are believed to be caused by prolonged hemodynamic stress.3,7 Posterior cerebral artery aneurysms are considered rare in occurrence, making up about 0.7% to 2.3% of all intracranial aneurysms.3,10
These pathological changes predispose patients to arterial rupture, potentially leading to a subarachnoid hemorrhage (SAH). Patients with SAH caused by posterior cerebral artery (PCA) aneurysms, though rare, often face a poor prognosis, despite advances in endovascular treatment.10 Only about 50% of patients survive an aneurysm rupture.7 The prognosis in these cases can be further complicated by the presence of other vascular anomalies, such as CCMs, which increase the risk of seizures, hemorrhage, or neurological deficits. CCMs, particularly when linked with developmental venous anomalies (DVAs), heighten the risk of hemorrhage.11,12
Cerebral cavernous malformation (CCM) was the other possible differential diagnosis for this patient. A CCM is considered a genetic vascular abnormality in the brain, consisting of small clusters of blood vessels that carry low blood pressure.4,5,8,11,13–15 They present as a single or mixed vascular lesion, and have different neurological implications if symptomatic.11 CCM’s, like PCAAs, are also rare, occurring in about 0.16% of the population. Additionally, CCMs can occur in 2 forms, sporadic and familial. The sporadic form is the most common form, accounting for most single lesions, and the familial form is a genetically inherited form caused by mutations to the CCM1(KRIT1), CCM2(MGC4607), or CCM3(PDCD10) genes.15,16 Seizures can occur in 50% of CCM cases, intracranial hemorrhage can occur in 25% of CCM cases, and focal neurologic deficits can occur in 25% of CCM cases. However, it is important to note that about 50-80% of CCM cases can be asymptomatic.12,14,16 The most concerning complication of CCM is symptomatic intracranial hemorrhage, making it the primary reason for treatment.13,17,18
Upon further questioning during a follow-up visit, the patient related that there was a possibility of an estranged blood relative having a CCM. The patient stated that this information came from a close family member, however there was no to prove or disprove this.
With this new information, one could speculate that he could indeed have a familial form of CCM. According to Zabramski’s classifications this patient has a presentation of a combination Type 1 and Type 4 CCMs due to a single lesion (2.1cm) in the medial right temporal lobe and small T2 hyperintense lesions within the right thalamus.5,19,20
The CCM classifications were published by Zabramski (J Neurosurg 1994;80[3]:422-432) where he describes CCM’s MRI signal characteristics and pathological characteristics, and classifies CCM’s into 4 different types.(Table 1)
Two studies, one by Toldo21 concluded that vertebral angiomas are associated with familial CCMs. A second study by Tandberg22 concluded that the simultaneous occurrence of intracranial and spinal cavernous angiomas in familial cerebral cavernous malformation (CCM) is very uncommon. They recommended providing comprehensive care for patients with familial CCM involving screening all potentially affected tissues, emphasizing the use of MRI in diagnosing spinal and vertebral cavernous angiomas in all patients with familial CCM and ensuring proper management by specialists.21,22
In this case, acknowledging that the coexistence of intracranial and spinal cavernous angiomas in familial CCM is exceedingly rare.21 The possibility cannot be completely ruled out, given the patient’s initial L-spine MRI revealed a L3 vertebral hemangioma combined with the imaging results resembling Zabramski’s criteria,19 and the possible family history of CCM. It would be feasible to side with the neuroradiologists of this case and provide this patient with a working diagnosis of familial CCM, pending genetic testing.
Due to the prolonged time lapse from onset to diagnosis, one could ask what would have been the course of intervention, surgical or not, if the diagnosis and prompt referral would have taken place soon after the onset of symptoms instead of two months later. As a result of the patient’s stable neurological symptomatology, his healthcare team cleared him to return to normal activity including weightlifting. In one study by Joseph23 where they observed no contraindications to aerobic physical activity, but due to limited evidence, they could not confidently recommend heavy lifting, contact sport, or activities with high changes in pressure like scuba diving. There remains the possibility of re-hemorrhage with such activities.23,24 The patient in this case was fortunate that the presenting neurological symptoms improved with time, without requiring surgical intervention, as such interventions are influenced by size and location of the lesion and neurological symptomatology.25 Hence, recognizing symptoms precisely and without delay is essential for the prompt management of these conditions.
Conclusion
This case highlights the need for primary care providers, including chiropractors acting as PCPs, to be vigilant when encountering neurological symptoms that may indicate a cerebrovascular event and as reminder of the importance of proper correlation of mechanism of injury, symptomatology, and physical exam to promptly recognize red flags and perform an accurate referral, especially in cases where brain involvement is suspected. We also highlight the importance of an accurate diagnosis to determine the ability for the patient to resume ADL’s, in this case weightlifting. There were differing opinions amongst clinicians throughout the management of this patient regarding the appropriate return to normal activity. There is still a difference in opinion between the doctors managing this case and thus the working diagnosis remains of that of a thrombosed PCA aneurysm, and a hemorrhaged CCM. After reviewing all the case details and reviewing the literature, a CCM seems more likely to be the patient’s diagnosis; however, it is impossible to achieve at such a conclusion without pertinent genetic testing. Further research is needed to better understand the impact of high-intensity physical activity on the risk of hemorrhage in individuals with CCMs or aneurysms, particularly in younger, physically active populations. After one year follow-up the patient has not had any bleeds reported or changes to neurological symptoms.
Acknowledgements
I would like to thank Dr. Yahaira Roman, DC, CCSP, Dr. Rishi Bodalia DC, MS, DACBR, and Dr. Mathew Richardson, DC, DACBR for their clinical contributions to the case presented in this report.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest
The author declares no conflict of interests.
Funding
Not applicable
_small_central_disc_protrusion_at_c6-c7_and_c7-t1(fig._1)__however__no_signif.jpeg)
_there_is_slight_t1_hypointense_signal_throughout_the_bone_marrow__though_thi.jpeg)

_small_central_disc_protrusion_at_c6-c7_and_c7-t1(fig._1)__however__no_signif.jpeg)
_there_is_slight_t1_hypointense_signal_throughout_the_bone_marrow__though_thi.jpeg)