INTRODUCTION
Instrument-Assisted Soft-Tissue Mobilization (IASTM) is a form of manual therapy involving rigid instruments of various shapes and materials to locate and treat soft tissue disorders.1–6 Although forms of IASTM have been used since ancient times using different materials, there has been a resurgence in its popularity, now using stainless steel instruments beginning with Graston Technique®. This has expanded to instruments made by other manufacturers in the past few years.4,5 The term IASTM does not differentiate between these various brands or techniques. IASTM is a non-invasive therapeutic technique which is usually applied by stroking the beveled edge of an instrument on the surface of the skin, often aided by lubricant, with the intent of influencing the underlying connective tissues, muscles and nerves.3–6 IASTM reportedly allows for the detection of altered tissue properties through increased vibration sense, while minimizing compressive forces to the interphalangeal joints of the clinician’s hands.3,6
While the therapeutic mechanism of IASTM is not clearly understood, there is some evidence that the technique may aid in the breakdown and absorption of scar tissue, mobilization of fascia, and improved tissue healing.5–9 Favourable effects on the organization of underlying collagen substructure have been reported, which may result in stronger, stiffer ligaments with an increased ability to absorb mechanical energy.8,9
IASTM has been hypothesized to play a neuromodulatory role in controlling pain by stimulating mechanosensitive neurons contained in the treated soft tissues including skin, muscle, and joint capsule.8–11 Compared to a clinician’s bare hands, the contact area of the IASTM instrument is significantly less, which is thought to lead to increased tensile and compressive stress.1,2 The skin deformation may lead to decreased activities of both large and small fiber neurons, which may in turn provide a form of analgesic response.12–15
To date there has been only 1 systematic review which appraised the effects of IASTM.16 That review evaluated papers which measured the effects of IASTM in several ways including range of motion (ROM), pain, balance, pressure sensitivity, strength, and self-reported functional outcomes. The review did not find evidence to support the efficacy of IASTM for treating musculoskeletal pathologies. They found weak evidence that IASTM may provide short term improvements in lower extremity joint ROM.16 The considerable heterogeneity in outcome measures in that review made it difficult to synthesize the results, make meaningful comparisons between papers, or draw conclusions about any specific effect or outcome measure.
The purpose of this systematic review is to assess whether IASTM is effective in reducing pain intensity in the treatment of musculoskeletal conditions.
METHODS
This systematic review was registered with the PROSPERO registry, number CRD42018118235 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews.17,18
Search Strategy
A systematic search strategy was designed and executed during the Fall of 2017 using the following databases: PubMed, PEDro, CINAHL, Medline, and SPORTDiscus. The search terms included the following terms, either individually or in combination: instrument, assisted, augmented, soft-tissue, mobilization, Graston, and technique. The terms Gua sha and ASTYM were omitted from this search. Although these techniques are similar to IASTM, the treatment rationale, outcome measures, and application differ from other IASTM techniques.19,20
Study Selection
The inclusion criteria for this review were as follows:
-
Peer reviewed, English language publications
-
Controlled clinical trials that studied the use of IASTM for musculoskeletal pain.
-
Studies that used pain severity as an outcome measure using instruments including but not limited to the Visual Analog Scale (VAS), Numeric Pain Rating Scale (NPRS), Verbal Rating Scale (VRS).
Studies were excluded if they included Gua sha and ASTYM, case reports, case series, clinical commentary, dissertations, conference posters, abstracts, or used outcome measures that were clinically unsuitable for the condition being treated. Studies that performed adjunctive or additional interventions were not excluded. While multiple interventions confound interpretation, the review team felt that the relative lack of literature justified their inclusion.
Two reviewers (AW and AK) independently evaluated the search results and selected studies for inclusion. After comparison, a third independent reviewer (KS) was available to resolve any disagreements.
Data Extraction and Quality Assessment
Two reviewers (AW and AK) extracted data independently from included studies and that information was tabulated. After comparison, a third reviewer (KS) was available to resolve disagreements. The following data were extracted from each article: research design, subject characteristics, condition treated, intervention parameters, and data pertaining to pre- and post-treatment pain severity, VAS change scores and between group differences as reported by the authors. The Minimal Clinical Important Difference (MCID) utilized in this review was 17mm on the VAS.21
Methodological quality assessment of selected papers was performed by 2 reviewers (AW and AK) using the PEDro (Physiotherapy Evidence Database) scale for appraising the quality of literature.22,23 A PEDro score of 6 or more is considered high quality evidence, while 4-5 is deemed to be fair quality, and 3 or less is poor quality.24 A Kappa statistic was calculated to determine the degree of agreement between the reviewers during methodological quality assessment.25 Interpretation of Kappa values, as described by Landis and Koch26 were as follows: <0 poor inter-rater agreement, 0.01 to 0.20 as slight agreement, 0.21 to 0.40 as fair agreement, 0.41 to 0.60 as moderate agreement, 0.61 to 0.80 as substantial agreement, and 0.81 to 1.0 as almost perfect agreement. A third reviewer (KS) was available in the case of disagreement.
RESULTS
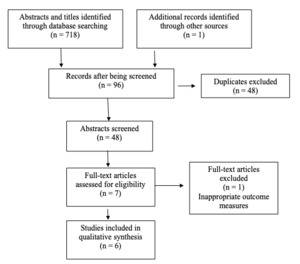
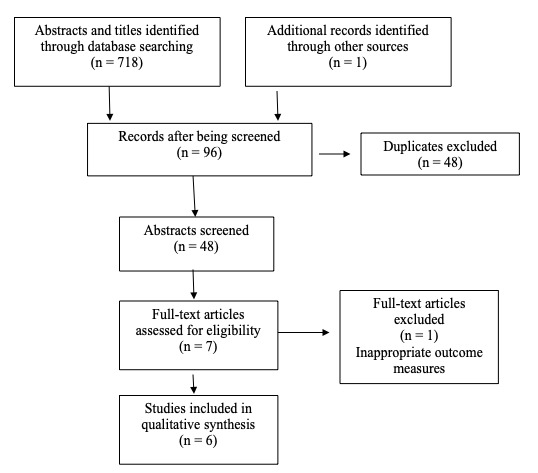
Figure 1 depicts the PRISMA flow diagram of articles through the review.17 A total of 719 records were identified after conducting the database searches, of which 671 were removed due to duplication or not meeting the inclusion criteria. A total of 48 abstracts were screened, and 8 full-text articles were obtained for further assessment. Following this, 5 randomized controlled trials27–31 and 1 controlled clinical trial 10 met the inclusion criteria for this review. One full-text was excluded because it did not appropriately report outcome measures for the region being treated.32 In this case, the intervention was applied to only the hamstrings; however, the visual analog scale (VAS) was being measured for low back pain intensity.
Methodological Quality Assessment
Table 1 provides the PEDro score for each of the included studies. All included studies scored 6 or higher on the PEDro scale, which is greater than the reported mean PEDro scores of musculoskeletal studies (5.08 ± 1.7) and manual sports therapy studies.33,34 The reviewer’s Kappa value for the 6 studies was 1.0.
Subject Characteristics
Table 2 summarizes the subject characteristics of the included studies. A total of 289 subjects participated across all 6 studies (164 male; 125 female). Four studies 27-29,31 analysed the effects of IASTM in the extremities (wrist, elbow, knee, ankle) while 2 studies 10,30 focused on thoracic and lumbar spine pain.
Interventions
Table 3 summarizes the results of the included studies. The Graston technique was reported as being the specific technique utilized in all studies. Only 3 studies10,28,31 reported the duration of each treatment dose. Five10,27,29–31 of the 6 studies compared IASTM to a non-IASTM group. All 5 of these studies demonstrated a statistically and clinically significant reduction in pain within the IASTM groups, except for the comparison group in Lee et al.10 and the experimental group in Schaefer et al.31 Only 427,29–31 of the 6 studies demonstrated a statistically significant change between groups.
The experimental group in the study by Blanchette and Normand27 received IASTM twice per week for 5 weeks, while the control group received advice on the natural history of lateral epicondylitis, computer ergonomics, and stretching exercises.
Brantingham et al28 utilized a multimodal approach for patellofemoral pain syndrome in which 1 group (Group A) received a combination of knee joint manipulation, IASTM, and exercise for a duration of 6 weeks. A second group (Group B) received the same IASTM and exercise, but replaced knee joint manipulation with manipulation to all joints in the kinetic chain from the lumbar spine to the foot.
Burke et al29 employed a combination of therapies for carpal tunnel syndrome (CTS) in which 1 group received IASTM to the affected forearm, wrist, and hand for 6 weeks. A second group received massage therapy to the same regions on the affected side. Both groups were prescribed upper-extremity home exercises.
Crothers et al30 examined the effects of spinal manipulative therapy (SMT) and IASTM on non-specific thoracic spine pain. One group received spinal manipulative therapy for the thoracic spine over 3-4 weeks; while a second group received IASTM to the posterior thoracic region and a third group received de-tuned ultrasound in the same region over the same length of time.
Lee et al10 assessed the management of low back pain where 1 group received IASTM to the lumbodorsal fascia, sacrum, lateral hip rotators and hamstring for 4 weeks while a second group was prescribed general exercise, stretching, stationary cycling for the same length of time.
Schaefer et al31 examined the effect of a combination of dynamic balance training (DBT), IASTM, and sham IASTM for chronic ankle instability. One group received DBT and IASTM to the ankle and foot, a second group received DBT and sham IASTM, while a third group received DBT only. All subjects received their respective intervention for 4 weeks.
Data Synthesis
The experimental group in Blanchette and Normand27 reported a significant decrease in pain on the VAS at both the 6-week (mean decrease of 30mm) and 3-month (mean decrease of 29mm) follow-ups, relative to baseline. The control group also reported a significant decrease in pain at the 3-month follow-up, with an average decrease of 18mm. As such, clinically significant change was demonstrated in both groups at 3 months.
In Brantingham et al,28 the average decreases in VAS-worst scores for both groups A and B were statistically significant, 19.5mm and 19.1mm, respectively. At the sixth treatment Group A showed statistically significant improvement in VAS-usual scores, but Group B did not. At the 2-month follow-up, the decrease in VAS-usual was statistically significant for group B (mean of 14.8mm), but not for group A (mean of 7.6mm). VAS-worst average changes were statistically significant and clinically meaningful for both groups at the 2-month follow-up, reporting a mean decrease of 20.4mm and 27.3mm in groups A and B, respectively. No significant difference was detected between groups from baseline to the 2-month follow up.
Immediately following both interventions in the study by Burke et al.29, there was a mean 48.7mm decrease in pain ratings in the IASTM group and an 18mm decrease in the control group. At 3-month follow-up, a slight increase in average pain ratings for subjects treated with massage was detected, while improvements in average pain ratings for the subjects treated with IASTM were maintained. Both the IASTM and comparison groups demonstrated statistically significant change; however, the reduction in pain was clinically significant at the three-month follow-up only in the IASTM group.
In Crothers et al,30 subjects in all 3 groups reported improvement in pain intensity from baseline to a 12-month follow-up. The decrease in VAS reported in the SMT and IASTM group from baseline to 12-month follow-up was 17mm. Similarly, the IASTM only group reported a mean decrease of 25mm. The placebo group decreased by an average of 22mm at the 12-month follow-up. All decreases were statistically and clinically significant.
In Lee et al,10 the IASTM group demonstrated a statistically and clinically significant decrease in pain by an average of 25.1mm from pre-intervention to post-intervention. The control group pre- to post-intervention VAS scores decreased by an average of 4.3mm, which was neither statistically nor clinically significant.
Schaefer et al31 demonstrated statistically significant reductions in pain across all three groups with average decreases of 14mm, 19mm, and 18mm in the experimental, control, and sham groups, respectively. Only the changes observed in the control and sham groups were considered clinically significant.
DISCUSSION
The purpose of this systematic review was to examine the use of IASTM as an intervention for the management of pain in musculoskeletal conditions. The only previous systematic review on this topic, Cheatham et al,16 measured the effects of IASTM in several different ways including range of motion (ROM), pain, balance, pressure sensitivity, strength, and self-reported functional outcomes. They found weak evidence for IASTM, due in part to the considerable heterogeneity in outcome measures in the evaluated papers. Our review aimed to assess the treatment effectiveness of IASTM solely on pain intensity.
Six studies10,27–31 were included in our review but varied in their study populations, methods, and outcome measures, thus preventing direct comparisons between studies. Five studies10,27–30 reported statistically and clinically significant reductions in pain within the IASTM groups, exceeding the MCID. However, none of the studies found clinically significant between-group differences.
There are several important factors to consider regarding the treatment interventions when interpreting treatment results. First, only 1 study 29 followed the recommended Graston® treatment protocol, which includes examination, warm-up, IASTM treatment, post treatment stretching, strengthening, and ice.3 However, we are not aware of any research to support the effectiveness of the recommended protocol as it is suggested by Graston’s developers. Additionally, the nature of the Graston treatment protocol itself creates uncertainty around concomitant treatment factors resulting in positive outcomes not necessarily attributable to IASTM. Second, it is difficult to compare the results of studies using only IASTM therapy versus those using IASTM as part of a treatment protocol with other adjunct therapies (e.g., ultrasound, stretching, manipulation, exercise, etc.). For example, Schaefer and Sandrey31 measured the effects of a 4-week dynamic balance program combined with IASTM on subjects with a history of chronic ankle instability. They found that pain improved in all groups with no significant difference between groups. However, the 3 groups in this study had several different interventions so it is difficult to isolate the effects of IASTM alone. Third, there were problems with heterogeneity of treatment times in the studies. Three studies10,28,31 reported varied treatment times ranging from 40 seconds to 8 minutes, with no clear pattern in the results. Three studies failed to report any treatment times.27,29,30
Results with respect to pain reduction varied among the selected studies. Five of the studies10,27–30 found some evidence for pain reduction using IASTM. Blanchette and Normand27 found a significant decrease in pain with grip strength in cases of lateral epicondylitis with the experimental group showing improvement faster than the control group. However, at 3-months the difference between groups was not significantly different. Brantingham et al28 compared 2 treatment protocols for patellofemoral pain syndrome. Although there were significant improvements in pain in both treatment groups (no significant difference between groups), it is difficult to draw conclusions about the efficacy of IASTM specifically given that it was included in both groups. Both groups in this study received similar IASTM interventions along with two different manipulation protocols and exercise. The improvements in both groups may have been due to any combination of IASTM, exercise and manipulation. Burke et al29 found a statistically significant improvement in pain in cases of carpal tunnel syndrome immediately following IASTM treatment and at 3-month follow-up. This study was the only 1 that followed the recommended Graston technique protocol. Lee et al10 found a statistically significant reduction in pain symptoms in patients with chronic low back pain following IASTM treatment. However, there were no between-group differences to compare as this study was a controlled clinical trial.
Limitations
There were several limitations in this systematic review, mainly due to the paucity and heterogeneity of evidence surrounding IASTM. It is challenging to assess IASTM effectiveness given the varied treatment times and application protocols in the various studies. A second limitation was the literature search only included English language publications, which may not have represented all of the available evidence from non-English studies or studies currently submitted for publication. A third potential limitation may be the search criteria focusing on IASTM methods using the most homogenous rationale and treatment approach, which led to the exclusion of Gua sha and ASTYM®.
Future Research
All studies included in this review had relatively small sample sizes ranging from 20 to 45 subjects; future studies with larger sample sizes would be useful. In addition, future studies should indicate more specifically the intervention protocols and in particular treatment time for IASTM. They should also further define the intervention protocol by stating if the Graston® protocol was followed or if just the instruments were used. Lastly, studies assessing IASTM in isolation rather than as part of a treatment protocol with other adjunct therapies would be useful.
CONCLUSION
The current state of literature surrounding the effectiveness of IASTM for pain in musculoskeletal conditions is unclear, although there is some evidence that warrants further investigation. Due to the paucity and heterogeneity of studies employing it, it is difficult to make meaningful clinical recommendations with respect to optimal IASTM programs, including dosage time, frequency, and type of instrument. Further studies are needed to assess different IASTM tools and protocols as the current evidence appears to lack the methodological consistency necessary to validate the effectiveness of IASTM itself or any of the IASTM protocols in the management of musculoskeletal pain.
ACKNOWLEDGEMENTS
The authors would like to express their sincere gratitude to Mr. Kent Murnaghan and Dr. Glenn Cashman for their continuous support and guidance.