INTRODUCTION
Osteoporosis is a disorder of decreased bone strength stemming from low bone mineral density (BMD) or impaired bone microarchitecture. As bone density decreases, fracture risk increases, often with low-trauma fracture of the spine, hip, proximal humerus, forearm, wrist, or pelvis. However, these underlying changes are initially asymptomatic, often going undiagnosed and untreated,1 until trauma facilitates diagnosis.
Globally, osteoporosis is by far the most common metabolic bone disease, affecting over 200 million people.2 In 2018, the National Osteoporosis Foundation found that 10.2 million adults in the United States have osteoporosis, with an additional 43.4 million having low bone mass (osteopenia).3 Further, approximately 50% of women and 20% of men over the age of 60 will experience a fracture that is attributable to osteoporosis.1
Studies have shown that in the year following a new osteoporotic fracture,15% of patients develop additional fractures and nearly 20% die. Among fractures from osteoporosis, hip fractures are the most dangerous, with a staggering 30% mortality within 12 months.4 Survival is also no guarantee of a return to health, with just over half of survivors permanently impaired, with many remaining bedridden.5 Worse still, annual osteoporotic hip fracture occurrence worldwide is projected to increase by five-fold by 2050.5
Current preventative paradigms for osteoporosis emphasize large supplemental doses of calcium and modest doses of vitamin D; however, these measures have a poor record of success.6 Pharmacological interventions favor bisphosphonates and estrogen, neither of which are recommended for long term use due to risks.1,7–9 The purpose of this paper is to summarize and discuss major themes associated with the diagnosis, treatment and prevention of osteoporosis.
METHOD
Literature searches via PubMed and Google Scholar were performed with the following key words: osteoporosis, osteopenia, bone mineral density, DXA, dual energy x-ray absorptiometry, bone markers, diet, exercise, hormonal impact, calcium, micronutrients, vitamin D, vitamin K, bone wasting medications, osteoporosis drugs, risk factors. In total, 188 human intervention trials and basic science articles were selected. All animal trials were excluded.
DISCUSSION
This review is structured to address 5 primary topics related to osteoporosis: etiology, diagnostics, risk factors, prevention and treatment, as well as general recommendations.
Etiology
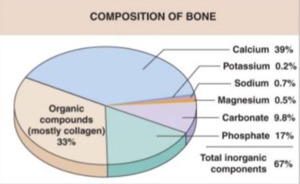
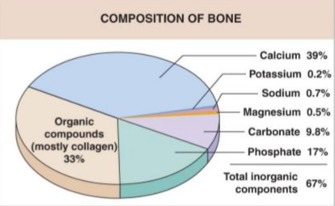
Human bone tissue consists of organic compounds (mostly collagen type-1 proteins), inorganic calcium hydroxyapatite (Ca10 (PO4)6 (OH)2), as well as smaller amounts of potassium, sodium, magnesium, and several other trace minerals.10 It is highly vascularized, metabolically active, and constantly responding to environmental stressors and mechanical stimuli.11,12 (Figure 1)
Bone constantly renews itself in a process called remodeling, which is carried out by 2 specialized cell types: osteoclasts and osteoblasts. Osteoclasts remove old, or damaged bone tissue, transferring the tissue components back into the bloodstream. Osteoblasts, under normal physiological conditions, will fill the small holes created by osteoclastic activity with a “mortar” of calcium, minerals, and collagen, forming new bone tissue.
A majority of bone mass is accrued in early adulthood, peaking around the age of 30 years.13 In a healthy adult, the rate of bone formation is roughly equivalent to the rate of bone resorption. However, in osteoporosis, the normal balance of osteoclast and osteoblast activity is altered resulting in accelerated resorption and/or decreased bone formation. As more tissue is resorbed than formed, bones become porous, increasingly fragile and vulnerable to fracture.
Osteoporosis can be classified into 2 distinct types. Primary osteoporosis is associated with normal aging and results from diminishing levels of male and female sex steroid hormones, which degrades the bone trabeculae and decreases mineral content.14,15 Secondary osteoporosis is osteoporosis caused by medical conditions or the use of medications that can interfere with bone reformation.16 Secondary osteoporosis can result from many causes, including disease, specific medication use, nutritional deficiencies, sedentary lifestyle, excessive alcohol consumption, and smoking. (Table 1) However, disorders of calcium metabolism (e.g., hyperparathyroidism, vitamin D deficiency) and bone-demineralizing medications, accounting for approximately 78% of the secondary causes.17
Diagnostics
Bone strength – here defined as the ability to withstand trauma without fracturing – is primarily a function of bone density. 75-90% of bone strength is directly related to bone mineral density (BMD).18 While BMD is not the only factor relating to bone strength, previous work has found that BMD is a quantifiable culmination of an individual’s history, genetics, risk factors, and treatment.19
Dual Energy X-ray Absorptiometry
Dual Energy X-ray Absorptiometry (DXA) is the most widely used method for measuring BMD and therefore is a major component of assessing fracture risk.20 Generally, DXA of the lumbar spine and proximal femur (which is subdivided to the femoral neck and total hip) are considered the gold standard measurements for BMD, as they not only quantify the ratio of bone formation to resorption, but also provide a normalized score that can be tracked over time.1,21,22 DXA is a specialized x-ray device that accurately measures bone mineral density; essentially the amount of calcium per area of bone. The results from a DXA test are expressed as T-scores, and are used by the World Health Organization (WHO), as well as the International Osteoporosis Foundation (IOF), to define osteoporosis (see Table 2).23 T-scores are a statistical representation of an individual’s BMD expressed in standard deviations difference from a sample of 20–29-year-old Caucasian females’ femoral neck measurement in the National Health and Nutrition Examination Survey (NHANES) III reference database.24 There is some controversy of these metrics, given the widespread application of female BMD measurements to their male counterparts.25,26 Regardless, the current international standard is to begin treatment (medication) for all patients with a T-score of less than -2.5.27 (Table 2)
Generally, the accuracy of DXA in calculating BMD is excellent; however, it is important to note that the presence of arthritic changes, especially at the lumbar spine, can falsely elevate resulting T-scores.28 However, DXA results at the lumbar spine can detect early bone loss, in patients over 64 years of age, the proximal femur is more reliable as degenerative changes of the lumbar spine can render the results unusable.29,30 In the event that lumbar and proximal femur DXA examinations are not possible (e.g. immobility, obesity, hip replacements) forearm measurements can also be used.31 Forearm measurements should also be used in the case of hyperparathyroidism, as this location will exhibit changes earlier than the lumbar and proximal femur (CITE).31
The need and/or frequency for repeat DXA tests is situational. Patients at low risk may only need long interval (5-10 years) follow up while patients at high risk and/or newly initiated treatment often are prescribed a 1-year repeat DXA. Intervals between tests are lengthened after a positive response to treatment.32
Other Diagnostics
In addition to DXA results, the fracture risk assessment tool (FRAX) is used to calculate the 10-year probability of fracture. The tool examines age, sex, weight, height, history of previous fracture, parental hip fracture, current smoking, use of glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol (3 or more units/day), and femoral neck BMD.33,34 However, Although the FRAX tool is simple to use, it is an extrapolation and estimation of a future fracture rather than a direct measurement of current bone density as is provided by DXA.
Trabecular bone scores (TBS) have also been used diagnostically. TBS is an indirect indicator of bone microarchitecture and attempts to predict fracture risk by assessing bone quality. Though it is a useful tool, it is recommended as a complement to BMD results, rather than a standalone assessment.35
Peripheral BMD measurements of the tibia and calcaneus are also possible using Quantitative Ultrasound (QUS). QUS devices directly measure the speed of transmission of sound through the bone being assessed and interpolate this to a T-score. These devices have not been found to be reliable for measuring yearly changes in BMD, however - and according to the WHO - QUS should not be used to diagnose osteoporosis.36
Laboratory Testing
If a patient’s DXA scan returns low T-scores, or there is suspicion of conditions affecting the skeleton, laboratory tests should be ordered. Laboratory testing can be thought of as existing in 2 related levels. First level testing can uncover secondary causes of decreased BMD. Indeed, if the first level tests are normal, approximately 90% of secondary osteoporotic causes can be ruled out. On the other hand, an abnormal first level lab warrants further, more specific, laboratory tests and investigation; this is second level testing.37 (Table 3)
Bone turnover markers are mainly used in clinical trials to monitor efficacy and response to therapy by assessing new bone formation or resorption and/or to evaluate patient adherence to treatments. The tests for new bone formation include osteocalcin, bone isoenzyme of alkaline phosphatase (B-ALP), and type I collagen peptides. The markers for bone resorption are urinary pyridinoline (PYR), urinary deoxypyridinoline (DPYR), and serum levels of type I collagen telopeptides (NTx, CTx).38,39 These are not used, nor recommended, as routine tests in the management of individual patients.
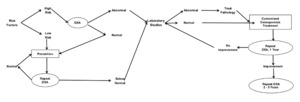
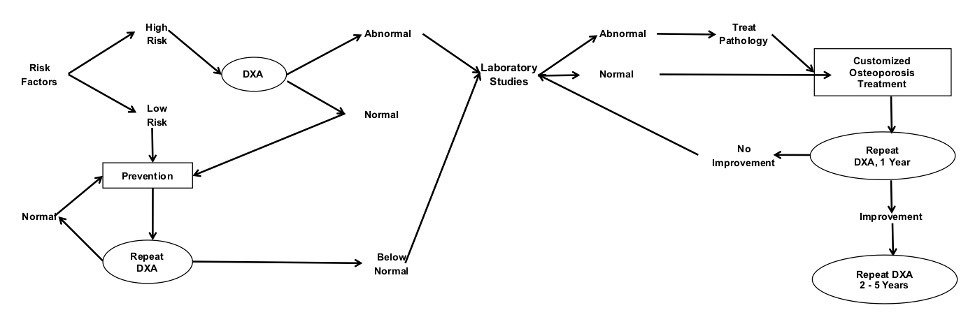
Figure 2 outlines a flow which providers can use to enhance their clinical diagnostics. The decision tree begins in the upper left-hand corner, by assessing a patient for risk factors. High risk individuals are screened using DXA testing. T-scores falling into pathological ranges can then be investigated with first and potentially second level laboratory tests. The information gathered from the DXA, and laboratory tests can then be used to tailor a personalized treatment program.
Risk Factors
Risk factors for osteoporosis can be categorized as either modifiable or non-modifiable.40–42 Modifiable factors are those that can be affected through lifestyle choices, therapeutic interventions and/or behavior. On the contrary, non-modifiable factors are mainly genetic or physiologic and are thus more difficult to affect. It is also important to note that risk factors, though important for clinical decision-making, do not accurately predict bone density.43
Modifiable Risk Factors
Medication and Drug Usage
There are a number of medications that can facilitate bone wasting (see Table 4), though it is important to note that the extent and severity of bone loss from these medications is dependent on dose and duration.44 However, of these medications, long term corticosteroid therapy is most commonly associated with drug-induced osteoporosis with the most rapid declines of bone mineral in the first 6 months of use.45,46
Progesterone-only birth control methods can also interfere in bone formation and resorption. Injectable Depo-Provera has been found to cause significant bone demineralization.47,48 Remineralization often occurs with cessation, however this is impacted by duration and age of use.49,50 Women closer to menopause are less able to rebuild bone density. Lower dose progesterone only contraceptives and combined estrogen-progesterone contraceptives have generally not been shown to suppress bone health.51
Diet
The consumption of high and ultra-processed foods and refined sugars, as well as inadequate protein and nutrient intake have been linked to osteoporosis. Ultra-processed foods are lacking in key nutrients required for bone health and often contain high-fructose corn syrup, hydrogenated oils, hydrolyzed proteins, or additives designed to increase flavor (such as flavor enhancers, colors, emulsifiers, emulsifying salts, sweeteners, and thickeners).52
In particular, these foods do not have adequate protein, fiber, vitamins A, C, D, and E, zinc, potassium, phosphorus, magnesium, and calcium, needed to support healthy bone.53 Similarly, the habitual consumption of high sugar drinks, such as soda, has been shown to be a risk factor for osteoporotic fractures.54,55 Sugar increases the urinary excretion of calcium, increasing inflammation, and hyperinsulinemia, hastening bone resorption.56
Adequate dietary protein is also essential for bone health. Approximately 50% of bone volume and 33% of bone mass is composed of structural proteins57; proteins are also involved in the secretion of bone-forming hormones, bone-related nutrient absorption and calcitriol synthesis.58
Inadequate nutrient intake has also been linked to osteoporosis and skeletal health. Vitamins B6, B9 (folic acid), B12, C, D, K are important for bone health, and deficiencies can lead not only osteopenia and osteoporosis, but also to disease states such as rickets and osteomalacia.59–61 Minerals such as calcium, boron, magnesium, manganese, strontium, zinc, and copper are also necessary for optimal bone health.62–67
Excessive alcohol consumption has direct and indirect effects on bone. Excessive is defined as more than two drinks per day for men and more than one drink per day for women.68 Alcohol often displaces good nutrition, has a toxic effect on osteoblasts, and increases the risk of falls. In contrast, modest consumption of alcohol has minimal or even a protective effect.68
Sedentary Lifestyle
Chronic unloading and/or weightlessness can lead to a rapid decline in bone density.69 Sedentary individuals are at risk of lower BMD due to the lack of mechanical stress placed on the bones.70,71 In the extreme, osteosarcopenia – the combination of bone and muscle wasting that can result from inactivity – increases the risk for both falls and fractures.72
Smoking
Smokers have twice the risk of osteoporotic fractures as non-smokers.73 Smokers have approximately 10% lower circulating levels of 1,25-dihydroxyvitamin D, the activated form of vitamin D.74,75 In a study of identical twins, where only 1 of twins smoked, bone mineral was approximately 10% lower. This is leading evidence that smoking is an independent risk factor for osteoporosis.76
Environment
Vitamin D deficiency is a global problem with approximately 42% of the US population being deficient.77 Sunshine (specifically ultraviolet B) striking exposed skin is the main source of vitamin D.78
For the same amount and time in sunshine, darker skinned individuals will absorb less UV radiation.79 The reason for this is the relative differences in skin melanin concentrations. Indeed, vitamin D deficiencies are more common in dark-skinned individuals.80,81 Studies investigating this phenomenon have found as many as 82% of African Americans suffering from hypovitaminosis D, followed closely by Hispanics at 70%.82
Certain environmental substances may increase osteoporosis risk. There is a possible link between osteoporosis and heavy metal exposure, such as cadmium (Cd) and lead (Pb), and industrial chemicals such as phthalates and per- and poly-fluoroalkyl substances (PFASs).83,84 However, these findings have been found in observational studies only and require further exploration.
Non-Modifiable Factors
Ethnicity
Bone density also varies among different ethnicities. Previous studies have found that Africans have higher BMD and lower fracture rates as compared with both Asians and Caucasians. Asians have an approximately 50% lower risk of a hip fracture than Caucasians.85,86
Genetics
Genetics have been found to account for 40-60% of peak bone mass. As greater lifetime peaks of bone density have been associated with lower risks of fracture over time, genetic influences play a significant role in the risk of osteoporosis and fracture.87–89
Age
For a given bone mineral density, fracture risk increases as bone quantity and quality deteriorates with age. In addition, decreases in muscle mass, strength, and balance (common issues associated with age) all increase fall and fracture risk.90,91 It is also important to note that a history of previous fragility fracture – a fracture from low energy trauma – is an independent risk factor for subsequent fractures and increasingly common with and indicative of osteoporosis.
Sex and Hormones
The single greatest contributor to bone mineral loss and fracture risk is decreased hormone levels. In women, the abrupt reduction in estrogen that occurs with menopause leads to increased osteoclastic and decreased osteoblastic activity, resulting in an acceleration of bone mineral losses.92,93
In early stages, this bone loss is found primarily in trabecular bone. Eventually, bone losses will be found in both trabecular and cortical bone.93 In the 4-8 years transition period to menopause, average BMD loss is 10%.93 Nearly half of women lose bone even more rapidly than this, with losses increasing up to 20%.94 This rate of loss can be compounded by earlier onset of menopause; the earlier the onset, the higher the risk of greater bone loss.95
In men, osteoporosis is underestimated and undertreated. Male fracture risk from osteoporosis is also related to decreasing hormone levels, though it presents with a different pattern of bone loss.96 Trabecular bone in men does tend to become thinner, but it maintains its connectivity better than in females.97 Fractures in men also occur 5-7 years later in life as compared with women98 and are often driven by secondary osteoporosis at approximately twice the rate of women.99,100 The risk of hip fracture in elderly men is 5-6% as compared with 16-18% for women. This translates to approximately 30% of hip fractures are being experienced by men.101
Prevention and Treatment
Diet
Dietary guidelines for the prevention of osteoporosis include eating whole foods, consuming adequate protein, having a diet with many dark green leafy vegetables, as well as avoiding excess sugar, alcohol, and smoking.102 Individuals should avoid heavily processed foods, and ensure they are achieving adequate vitamin and mineral intake from their diet.103 Recommended protein intake is 1.0-1.2 g/kg body weight/day.104
Calcium is undeniably important for bone health. The recommended daily allowance (RDA) for calcium for adults is 1000-1200 mg/day, though some studies have disputed this amount, suggesting instead a practical allowance of 400-500 mg/day.105 Several studies have even found that less than 300 mg/day will still confer benefits.106,107 Good dietary sources of calcium include kale, sardines, milk and cook beans, and can provide 100-350 mg/serving (e.g. 1 cup cooked collards or kale, 3 ounces of sardines, 8 ounces of milk, or 4 ounces of cooked beans). Many foods can supply or surpass the RDA (see Appendix 1).
Overconsumption of calcium does not appear to add any measurable benefit; however, with deficiency, supplementation is advantageous.108,109 Previous literature has found a strong correlation between calcium, dairy consumption, and bone health.110,111 Interestingly, though calcium is undeniably a component of bone, numerous trials have found little or no correlation between calcium (or dairy) consumption and bone mineral density and/or rate of fractures.108,112–114
Calcium is not always readily available in every diet and, as such, supplementation is advised. There are numerous, conflicting, opinions as to the preferred form of calcium supplementation. Considerations must include solubility, molecular size, pill size, particle size per manufacturer, as well as cost. Table 5 displays some of the benefits and drawbacks of four commonly available forms.
Calcium citrate appears to be the best choice when considering solubility, versatility, and cost. There are other forms of calcium supplements beyond the four listed above; hydroxyapatite, citrate-malate, acetate. None of these seem to have any advantage over citrate.115
Beyond bioavailability, calcium absorption is itself multifactorial. Optimal levels of Vitamin D can facilitate greater calcium absorption, as well as taking calcium with other foods and in smaller doses over a greater number of meals. Pregnancy has also been found to increase calcium absorption rates. Conversely, foods containing oxalates and/or phytates, low serum vitamin D levels and hypo or achlorhydria can decrease calcium absorption.116
Though the RDA for vitamin D is 400-800 IU/day, there is growing evidence that this may not be enough.117–121 Levels of Vitamin D are quantified by measuring blood concentrations of 25,OH-vitamin D (see Table 6).
It is important to note, however, that calcium and vitamin D have both received disproportionate attention when discussing diet. While both compounds contribute to skeletal health, neither is enough to address the wide variety of components necessary to build and maintain bone.1,122 Vitamins C and K, as well as B6, B9 and B12 are also important for bone health.123–125
Vitamin C appears to influence bone health in several ways. Vitamin C suppresses osteoclast activity, acts as a co-factor for osteoblast differentiation, enhances intestinal calcium absorption, and is needed for collagen formation.126 Adequate intake is positively associated with higher BMD127 as well as lower rates of hip fracture.128 Vitamin C can be found in fresh produce (see Appendix).
Vitamin K, especially K2M7, has been shown to have a synergistic effect with vitamin D, further promoting bone health.129,130 Vitamin K is multifunctional and has been demonstrated to promote bone formation by stimulating osteoblast differentiation and inhibiting osteoblast apoptosis,131 regulating extracellular matrix mineralization,132 and decreasing bone resorption by inhibiting osteoclast differentiation.131 Vitamin K has also been shown to induce and activate osteocalcin (bone GLA protein),133–135 a compound that binds calcium ions to hydroxyapatite crystals.132,136 Providers should also be aware that the use of statin drugs can inhibit the formation of vitamin K-dependent proteins, specifically the activated form of osteocalcin; carboxylated osteocalcin (cOC).137 Clinical trials using vitamin K in isolation yielded small, statistically insignificant, increases in bone mineral and minimal fracture reduction.138,139 However, combining vitamins D and K [as K2M4140 and K2M7141] yield positive, statistically significant improvements.
The RDA for magnesium is 310-360 mg and 400-420 mg for women and men, respectively.142 Approximately 50–60% of the total body magnesium content is accumulated in bone, and magnesium deficiency has been associated with reduced bone formation.143–145 Magnesium is also a co-factor needed for the activation of vitamin D.146 However, though there is leading evidence for an important role for magnesium in osteoporosis, no randomized studies have evaluated the effects of magnesium in isolation, and there is only a weak association between low serum magnesium levels and osteoporosis.147,148
Several trials demonstrate the benefits of trace minerals including magnesium, manganese, zinc, boron, and strontium.149–155 Table 7 provides RDA values for these minerals.
Compounds other than protein, vitamins and minerals have also received research attention. Collagen, Cissus quadrangularis, soy isoflavones, ipriflavone, DHEA, melatonin, and dried plums (prunes) have all been examined. Small and/or short duration studies looking at the effects of collagen156,157 and Cissus quadrangularis158 show encouraging results, though longer-term studies are needed.
In contrast, several well-done studies using soy isoflavones, ipriflavone, DHEA, and dried plums have demonstrated consistent benefit for bone health.159–162 Therapeutic doses of both soy isoflavones and ipriflavone should be restricted to use in women due to their estrogenic properties. DHEA is an adrenal hormonal which can be converted into a number of sex steroid hormones. Though it has inherent potency, more research is needed to understand all of its effects at varied doses. Dried plums have been subjected to several trials with sound methodology, yielding positive results. Studies have found leading evidence that consumption can increase bone formation, increase trabecular microstructure, decrease osteoclastogenesis, and decrease RANKL (a compound leading to bone resorption).163–168
Melatonin appears to both enhance anabolic effects and have antiresorptive benefits. Melatonin improves bone formation by promoting the differentiation of mesenchymal cells into osteoblasts. Bone resorption is reduced by diminishing the synthesis of RANKL.169,170
Combinations of micronutrients also appear to offer greater benefit than single nutrient treatments for osteoporosis. The Combination of Micronutrients for Bone Study (COMB) investigated 77 osteoporotic patients who either declined or failed with a trial of pharmacotherapy. These patients were offered a nutrient combination that included vitamin D3, vitamin K2, strontium, magnesium, and fish oil derived DHA (docosahexaenoic acid). After 12 months, the repeat DXA demonstrated statistically significant improvement of BMD at the spine, hip, and femoral neck sites. There were no fractures in the treatment group.171
The Melatonin-micronutrients Osteopenia Treatment Study (MOTS) was a 1-year double-blind randomized controlled trial using a combination of melatonin, strontium, vitamin D3, vitamin K2 (MK-7) that measured bone mineral, bone markers, and quality of life for postmenopausal osteopenic women. The treatment group had significant increases in BMD which were mirrored by changes in numerous markers of increased bone formation and decreased bone resorption. The intended improvements in skeletal health were accomplished in addition to benefits in mood and sleep quality. This well-designed combination of micronutrients appears to offer an effective treatment option and can be safely used with osteopenic patients, prior to further declines in BMD.172
Neither the COMB nor MOTS study described any adverse events or unintended consequences during the one-year duration of the trials. Though safety beyond one year duration is unclear, these nutrient combinations appear to be safe and effective within that time frame. Not all combination supplement trials show benefit.173
Medications (Table 8)
The list of prescription treatments has grown substantially since 1995, when the first osteoporosis-specific medications were approved by the United States Food and Drug Administration (USFDA). These medications can be categorized either by hormone vs. non-hormone, antiresorptive vs. anabolic, oral vs. injection, or first line vs. second line.
However, compliance with these medications can be poor, with 20-30% of patients stopping treatment within 6-12 months.174,175 Reasons for non-compliance include both the actual side effects as well as the fear of reported risk.174
Exercise
Exercise-based therapies should seek to safely increase skeletal loading and stimulate positive osseus adaptations to mechanical stress. The overall goal of training should be to reduce the risk of falls and improve strength and mobility.176 Walking has been found to increase muscle tone and overall fitness, as well as reduce the number of fall events, though only shown minimal changes in BMD.177 Resistance and higher impact training paradigms such as weightlifting and running offer greater benefits to BMD, while also facilitating strength and balance.178,179
Yoga,180 tai chi,181 unipedal standing,182 and walking,177 though less beneficial for BMD than impact exercise, have shown significant value for overall health, balance, and fracture reduction.
Some studies have suggested that vibration of the whole body or regional areas offers potential benefit for bone formation; however, these studies have differing protocols, used various endpoints, and were relatively short term.183,184 As such, the parameters of the therapy and results of care have been inconsistent.
Summary of Recommendations
Numerous professional groups have put forth guidelines germane to the diagnosis, prevention and treatment of osteoporosis. Guidelines from the American Association of Clinical Endocrinologists, American College of Endocrinology, North American Menopause Society, Endocrine Society, American College of Rheumatology, National Osteoporosis Foundation, and the American College of Physicians are all relatively consistent and focus almost entirely on treatment with medication.185
It is important to note that a single treatment for all osteoporotic patients is unrealistic given varied etiologies. Potential treatments and preventive protocols should be seen as guidelines that require personalization. The intervention for a 16-year-old with anorexia, a 25-year-old elite athlete or an 80-year-old senior cannot be identical. The rate of BMD decline is variable, and is influenced by genetics, hormonal status, nutrition, exercise, some disease processes, and medications.45 Treatments can – and should – be as numerous and comprehensive as the number of risk factors for this disease and take sex, activity level, lifestyle and comorbidities – at a minimum – into account.
Gold standard testing and diagnosis currently utilizes guidelines established by the WHO based on DXA testing. T-scores from DXA testing provide adequate summation of the spectrum of factors that can influence bone health (e.g. genetic, lifestyle, medications, exercise, etc.), but does not specify the underlying reason(s). Additionally, examination of T-scores over time provide a means to evaluate trending and responses to treatment. Follow-up DXA testing should be as dynamic as skeletal health and based on an individual’s unique presentation. In an older adult with a T-score necessitating treatment, follow up in 1-year is warranted. If the follow up DXA demonstrates an increase in BMD after the initiation of treatment, then greater intervals between tests is appropriate. In a patient with low risk and a normal DXA result, a repeat test in 5-10 years is reasonable. Providers should note that there are known shortcomings with these criteria – specifically that the normative values T-scores are based on rely entirely on a sample of under 30-year-old Caucasian women24 and thus may be less accurate when approximating BMD in men, as well as both sexes of other ethnicities. Laboratory tests can also be helpful in identifying secondary causes of osteoporosis (such as hyperparathyroidism, vitamin D deficiency or kidney disease) following an unexpectedly low DXA test. If laboratory tests are abnormal, further testing is often indicated. A battery of both DXA and laboratory examinations can be used to create an individualized treatment program.
Prevention and treatment strategies for osteoporosis should begin with a balanced nutritious diet. General dietary guidelines for the prevention of osteoporosis include eating whole foods, consuming adequate protein, having a diet with many dark green leafy vegetables, as well as avoiding excess sugar, alcohol, and smoking.186 Individuals should avoid heavily processed foods, and ensure they are achieving adequate vitamin and mineral intake from their diet.103 Though calcium and vitamin D are important to bone health, they are far from the only vitamins and minerals needed. Supplements should be considered in the event that diet alone cannot supply all of the needed bone building nutrients. It is also important to note that vitamins and minerals do not operate independently; rather, the absorption and utilization of one may rely entirely on the presence of another. Dosage, schedule of intake and bioavailability are all important factors to consider in designing a nutritional intervention. (Table 10).
Nutrient interventions can be categorized into diet, supplementation to insure quantities consistent with the USRDA, and pharmacologic doses. Diet sources and RDA quantities are associated with minimal risk. The risk: benefit relationship must be considered when prescribing any mega or pharmacologic doses or combinations. Additionally, nutrient-drug interactions may also impart risk or additional benefits.
Table 10 is a composite of lifestyle and supplements shown to enhance bone health. To date, there is no single study using the entirety of the summary table. Rather, this list expands upon the successful COMB and MOTS trials.171,172
Exercise based on increased skeletal loading and impact can help to build and maintain bone. Exercises such as volleyball, dancing, and weight training are all helpful for bone formation.187 Walking and swimming are excellent cardiovascular exercises, but less helpful for creating the forces necessary to stimulate bone formation. There is indirect evidence for yoga and tai chi in maintaining muscle mass and balance as well as reducing fall risk.
CONCLUSION
The literature is limited on the subject of manual therapy risk or benefit for osteoporotic patients. However, in regard to safety, one review concluded that Grade II to IV mobilization techniques were used with no serious adverse events reported.188 Providers should use best judgment with treatment recommendations.
The adaptive process of assessing risk, DXA and laboratory testing, prescribing dietary/supplement/exercise/lifestyle interventions that are customized to individual patient’s needs requires more provider effort, but in the end will yield more positive outcomes than management limited to medications or single nutrients.